Abstract
Background/Introduction: Bruton tyrosine kinase inhibitors (BTKi) are a mainstay of treatment for B-cell malignancies; however, their use can be limited by adverse events (AEs), many of which are potentially caused by off-target inhibition of other tyrosine kinases. The next-generation BTKi zanubrutinib was designed to maximize tolerability by minimizing off-target binding. Previous results from this ongoing phase 2 study (BGB-3111-215; NCT04116437) showed that zanubrutinib is well tolerated in pts intolerant to ibrutinib and/or acalabrutinib (Blood 2021;138[suppl 1]:1410). Here, we report updated results of the tolerability and efficacy of zanubrutinib in pts intolerant to acalabrutinib (cohort 2).
Methods: Eligible pts with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), Waldenström macroglobulinemia (WM), mantle cell lymphoma (MCL), or marginal zone lymphoma (MZL) who met protocol-defined criteria for intolerance to acalabrutinib received zanubrutinib 160 mg twice daily or 320 mg once daily. Pts who progressed on prior BTKi therapy were excluded. Safety and efficacy, including recurrence of acalabrutinib intolerance events, were evaluated. Investigators assessed responses every 3 cycles based on standard response criteria for each indication using parameters at study entry as baseline.
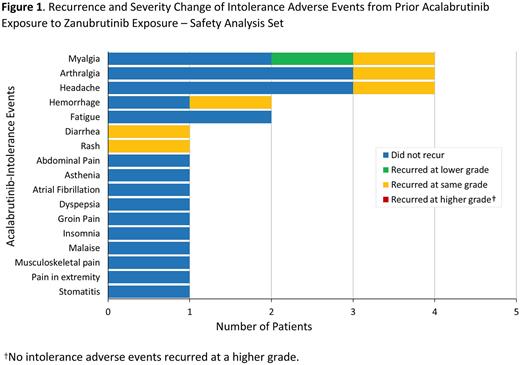
Results: As of June 6, 2022, 17 pts received zanubrutinib in cohort 2 (12 CLL/SLL; 3 WM; 1 MCL; 1 MZL). Median age was 74 y (range, 51-87); median treatment duration was 9.2 mo (range, 0.5-20.9), with median follow-up of 10.4 mo (range, 1.1-20.9). Median number of prior therapies was 2 (range, 1-6); 9 (53%) pts received prior ibrutinib and acalabrutinib. Median cumulative exposure to acalabrutinib was 3.8 mo (range 0.5-26.9). Twelve pts remain on treatment and 5 discontinued treatment (adverse event [AE] n=2, withdrawal n=2, PD n=1). A total of 28 acalabrutinib-intolerance events were reported in the 17 pts, most commonly arthralgia, myalgia, and headache (4 each) as well as hemorrhage and fatigue (2 each). Twenty-one (75%) acalabrutinib-intolerance events did not recur on zanubrutinib, corresponding to 11 (65%) pts not experiencing any recurrence of intolerance. Seven events (25%) recurred (1 at a lower grade, 6 at the same grade, 0 at a higher grade; Figure 1) and 2 pts discontinued due to recurrence (myalgia and diarrhea; both recurred at same grade). Two pts who experienced the same intolerance event (pain in extremity and atrial fibrillation) on ibrutinib and acalabrutinib did not have a recurrence of those on zanubrutinib. Although many patients entered the study with well controlled disease, among the 14 efficacy-evaluable pts on zanubrutinib, 13 (93%) achieved at least stable disease and 9 (64%) achieved a deepening of response.
Conclusion: With a median zanubrutinib exposure that was longer than the reported cumulative acalabrutinib exposure before discontinuation (9.2 mo vs 3.8 mo, respectively), disease was controlled in 13 (93%) of 14 efficacy-evaluable pts treated with zanubrutinib, and 11 (65%) did not experience any recurrence of their prior acalabrutinib intolerance events. This suggests that pts intolerant to acalabrutinib can attain clinical benefit by switching to zanubrutinib. Enrollment and follow-up are ongoing.
Disclosures
Shadman:MEI Pharma: Consultancy; Fate Therapeutics: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; Innate Pharma: Consultancy; Epi Lilly: Consultancy; Adaptimmune: Consultancy; AstraZeneca: Consultancy, Research Funding; Epizyme: Consultancy; Mustang Bio: Consultancy, Research Funding; Beigene: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Regeneron: Consultancy; Merck: Consultancy; Adaptive Biotechnologies: Consultancy; Morphosys/Incyte: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Sound Biologics: Consultancy; Kite Pharma: Consultancy; Abbvie: Consultancy, Research Funding; Atara Biotherapeutic: Consultancy, Research Funding; Celgene, a BMS Company: Research Funding; Gilead: Research Funding; Sunesis: Research Funding; Genmab: Research Funding. Flinn:Myeloid Therapeutics: Research Funding; IGM Biosciences: Research Funding; Genmab: Consultancy; BeiGene: Consultancy, Research Funding; Nurix Therapeutics: Consultancy, Research Funding; Loxo@Lilly: Research Funding; Merck: Research Funding; Pfizer: Research Funding; Xencor: Consultancy; Janssen: Consultancy, Research Funding; Epizyme: Research Funding; Takeda: Consultancy; Servier Pharmaceuticals: Consultancy; Roche: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Kite Pharma: Consultancy, Research Funding; Vincerx Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Verastem: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; Unum Therapeutics: Research Funding; Rhizen Pharmaceuticals: Research Funding; Acerta Pharma: Research Funding; Agios: Research Funding; ArQule: Research Funding; Celgene: Research Funding; MorphoSys: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Seattle Genetics: Research Funding; InnoCare Pharma: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Century Therapeutics: Consultancy; Biopath: Research Funding; Secura Bio: Consultancy; Forty Seven: Research Funding; Constellation Pharmaceuticals: Research Funding; Curis: Research Funding; CALGB: Research Funding; Portola Pharmaceuticals: Research Funding; Bristol Myers Squibb: Research Funding; Incyte: Research Funding; Infinity Pharmaceuticals: Research Funding; Hutchison MediPharma: Consultancy; Gilead Sciences: Research Funding; CTI Biopharma: Research Funding; City of Hope National Medical Center: Research Funding; CALIBR: Research Funding; Trillium Therapeutics: Research Funding; Forma Therapeutics: Research Funding; Fate Therapeutics: Research Funding; AstraZeneca: Consultancy, Research Funding; Iksuda Therapeutics: Consultancy; Millenium Pharmaceuticals: Research Funding; Tessa Therapeutics: Research Funding; TCR2 Therapeutics: Research Funding; 2seventy bio: Research Funding; Triphase Research & Development Corp: Research Funding. Levy:Jazz Pharmaceuticals: Honoraria, Speakers Bureau; Abbvie: Honoraria, Speakers Bureau; Seagen: Honoraria, Speakers Bureau; BeiGene: Honoraria, Speakers Bureau; TG Therapeutics: Honoraria, Speakers Bureau; Gilead: Honoraria, Speakers Bureau; AstraZeneca: Honoraria, Speakers Bureau; Epizyme: Honoraria, Speakers Bureau; Karyopharm: Honoraria, Speakers Bureau; Sanofi: Honoraria, Speakers Bureau; GSK: Honoraria, Speakers Bureau; Sellas: Membership on an entity's Board of Directors or advisory committees; Baylor University Medical Center: Current Employment; Amgen: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; Morphosys: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Dova: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau. Holmes:Seattle Genetics: Speakers Bureau; Rigel: Consultancy, Speakers Bureau; Novartis: Consultancy, Research Funding; Kite: Consultancy, Honoraria, Research Funding, Speakers Bureau; Karyopharm: Consultancy, Speakers Bureau; Janssen: Consultancy; Incyte: Research Funding; Genentech: Research Funding; Exuma Biotech: Membership on an entity's Board of Directors or advisory committees, Research Funding; Epizyma: Consultancy; Crispr Biosciences: Consultancy; Caribou Biosciences: Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Autolus: Research Funding; Astra-Zeneca: Consultancy; Artiva: Research Funding; Adicet Bio: Research Funding; TG Therapeutics: Consultancy; C Therapeutics: Consultancy, Research Funding. Farber:MorphoSys/Incyte: Other: Travel, Accommodations, Expenses, Speakers Bureau; Seagen: Other: Travel, Accommodations, Expenses, Speakers Bureau; Kite/Gilead: Other: Travel, Accommodations, Expenses, Speakers Bureau; ADT Therapeutics: Other: Travel, Accommodations, Expenses, Speakers Bureau; Genentech: Other: Travel, Accommodations, Expenses, Speakers Bureau; BMS: Honoraria. Chaudhry:Summit Cancer Center: Current Employment. Crescenzo:SAGA Diagnostics: Current equity holder in private company; GSK: Current equity holder in publicly-traded company; Pfizer: Current equity holder in publicly-traded company; BeiGene: Current Employment, Current equity holder in publicly-traded company. Idoine:BeiGene: Current Employment, Current equity holder in publicly-traded company. Zhang:BeiGene: Current Employment, Current equity holder in publicly-traded company. Cohen:BeiGene: Current Employment, Current equity holder in publicly-traded company. By:BeiGene: Current Employment. Huang:BeiGene: Current Employment, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding. Sharman:BMS: Consultancy, Research Funding; Genentech: Consultancy; TG Therapeutics: Consultancy, Research Funding; Merck: Consultancy; Lilly: Consultancy, Honoraria, Research Funding; Araris Biotech AG: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Consultancy, Honoraria, Research Funding; Pharmacyclics LLC, an AbbVie Company: Honoraria; Beigene: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal